Unit 2: The Chemistry of Water
Unit Outline
Part 1. Biological importance of water
- Water as a Lubricant and Cushion
- Water as a Heat Sink
- Water as a Component of Liquid Mixtures
- The Role of Water in Chemical Reactions
Part 2. Fluid compartments in the human body
Practice Questions
Learning Outcomes
At the end of this unit, you should be able to:
I. Explain the biological importance of water.
II. Describe the distribution of body water.
III. Describe the electrolyte composition of the body.
IV. Define and describe the measurement of pH.
As much as 70 percent of a human’s body weight is water. This water is contained both within the cells and between the cells that make up tissues and organs. Its several roles make water indispensable to human functioning.
Part 1. Biological Importance of Water
Water as a Lubricant and Cushion
Water is a major component of many of the body’s lubricating fluids. Just as oil lubricates the hinge on a door, water in synovial fluid lubricates the actions of body joints, and water in pleural fluid helps the lungs expand and recoil with breathing. Watery fluids help keep food flowing through the digestive tract, and ensure that the movement of adjacent abdominal organs is friction free.
Water also protects cells and organs from physical trauma, cushioning the brain within the skull, for example, and protecting the delicate nerve tissue of the eyes. Water cushions a developing fetus in the mother’s womb as well.
Water as a Heat Sink
A heat sink is a substance or object that absorbs and dissipates heat but does not experience a corresponding increase in temperature. In the body, water absorbs the heat generated by chemical reactions without greatly increasing in temperature. Moreover, when environmental temperature soars, the water stored in the body helps keep the body cool. This cooling effect happens as warm blood from the body’s core flows to the blood vessels just under the skin and is transferred out to the environment as radiant heat. At the same time, sweat glands release warm water in sweat. For evaporation of this water to occur, the hydrogen bonds between the water molecules must be broken, requiring a relatively high amount of energy that in part includes heat. This removal of heat by evaporation results in a cooling of the blood in the body’s periphery, near the surface of the skin, which then circulates back to the body core and cools the body.
Water as a Component of Liquid Mixtures
A mixture is a combination of two or more substances, each of which maintains its own chemical identity. In other words, the constituent substances are not chemically bonded into a new, larger chemical compound. The concept is easy to imagine if you think of powdery substances such as flour and sugar; when you stir them together in a bowl, they obviously do not bond to form a new compound. The room air you breathe is a gaseous mixture, containing argon, molecules of nitrogen and oxygen, and one compound— carbon dioxide.
For cells in the body to survive, they must be kept moist in a water-based liquid called a solution. In chemistry, a liquid solution consists of a solvent that dissolves a substance called a solute. An important characteristic of solutions is that they are homogeneous; that is, the solute molecules are distributed evenly throughout the solution. If you were to stir a teaspoon of sugar into a glass of water, the sugar would dissolve into sugar molecules separated by water molecules. The ratio of sugar to water in the left side of the glass would be the same as the ratio of sugar to water in the right side of the glass. If you were to add more sugar, the ratio of sugar to water would change, but the distribution—provided you had stirred well—would still be even.
The Role of Water in Chemical Reactions
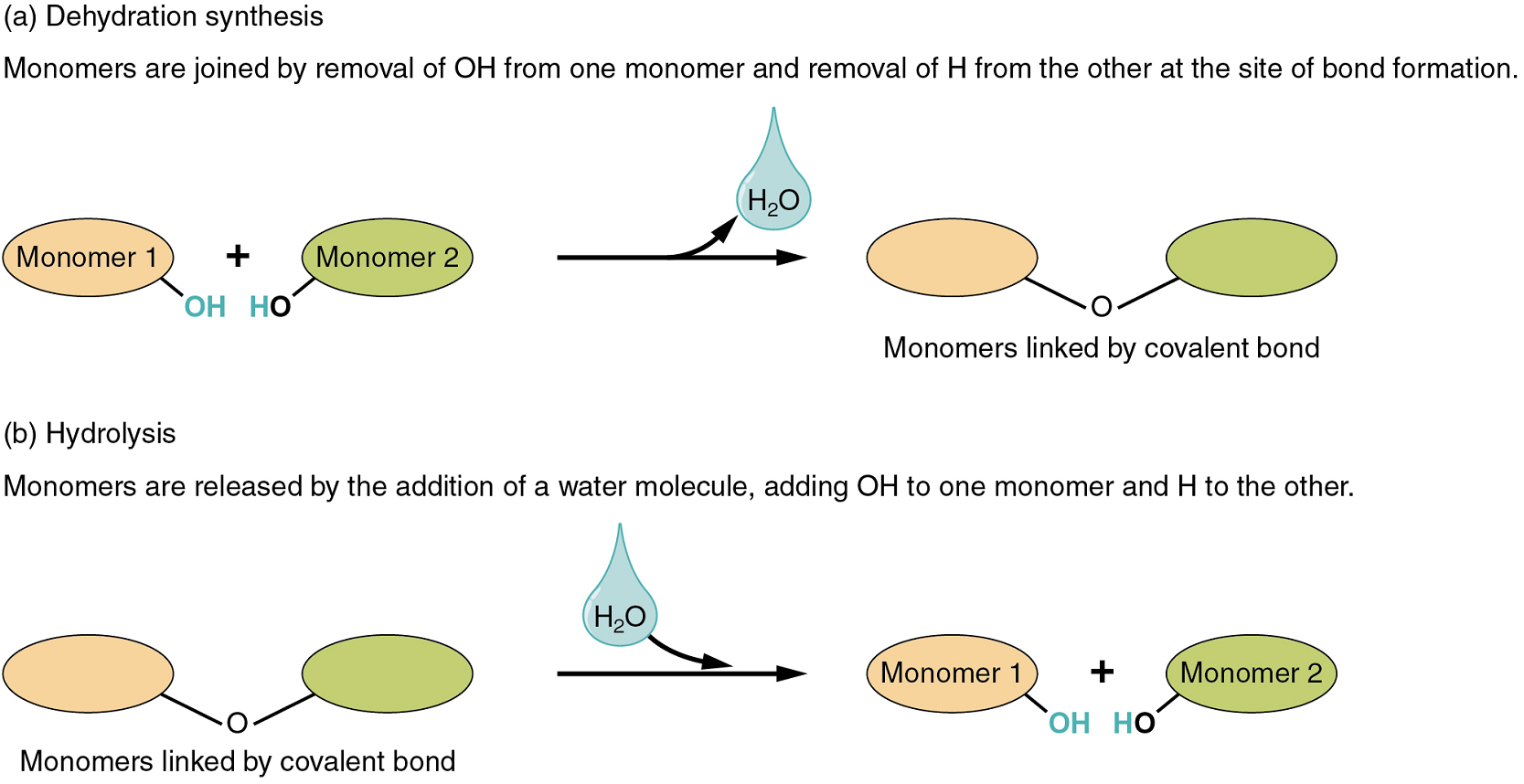
Two types of chemical reactions involve the creation or the consumption of water: dehydration synthesis and hydrolysis.
- In dehydration synthesis, one reactant gives up an atom of hydrogen and another reactant gives up a hydroxyl group (OH) in the synthesis of a new product. In the formation of their covalent bond, a molecule of water is released as a byproduct (Figure 1). This is also sometimes referred to as a condensation reaction.
- In hydrolysis, a molecule of water disrupts a compound, breaking its bonds. The water is itself split into H and OH. One portion of the severed compound then bonds with the hydrogen atom, and the other portion bonds with the hydroxyl group.
These reactions are reversible, and play an important role in the chemistry of organic compounds (which will be discussed shortly).
Part 2. Fluid Compartments in the Human Body
Body Water Content
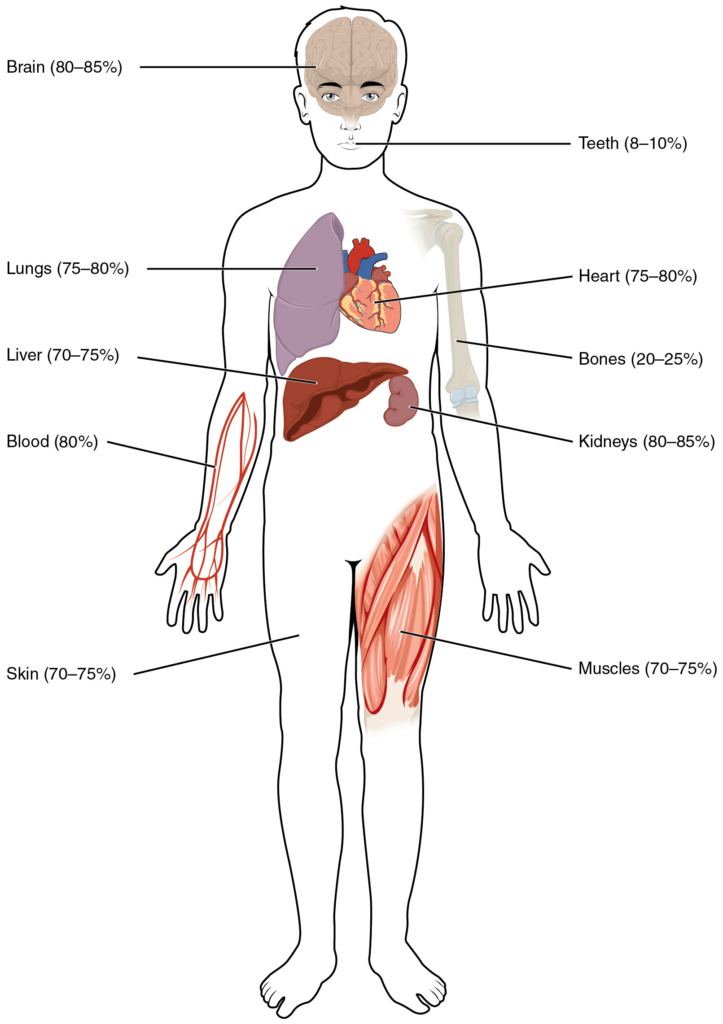
Human beings are mostly water, ranging from about 75 percent of body mass in infants to as low as 45 percent in old age. In adults, the average percent of body mass in women is 50 percent, whereas in men the average is 60 percent. The percent of body water changes with development, because the proportions of the body given over to each organ and to muscles, fat, bone, and other tissues change from infancy to adulthood (Figure 2).
Fluid Compartments
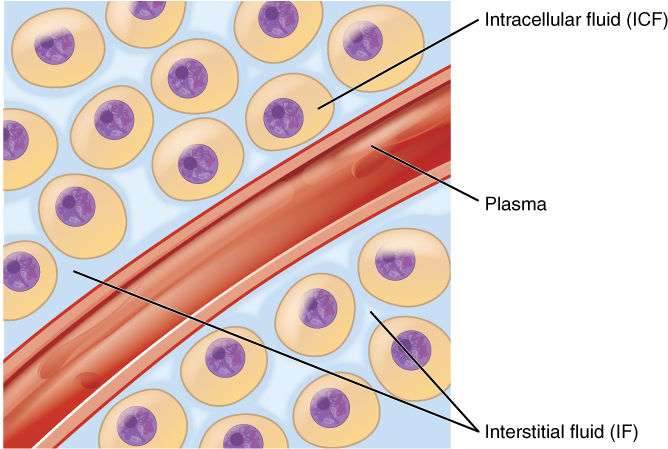
Body fluids can be discussed in terms of their specific fluid compartment, a location that is largely separate from another compartment by some form of a physical barrier. The intracellular fluid (ICF) compartment is the system that includes all fluid enclosed in cells by their plasma membranes. Extracellular fluid (ECF) surrounds all cells in the body. Extracellular fluid has two primary constituents: the fluid component of the blood (called plasma) and the interstitial fluid (IF) that surrounds all cells not in the blood (Figure 3).
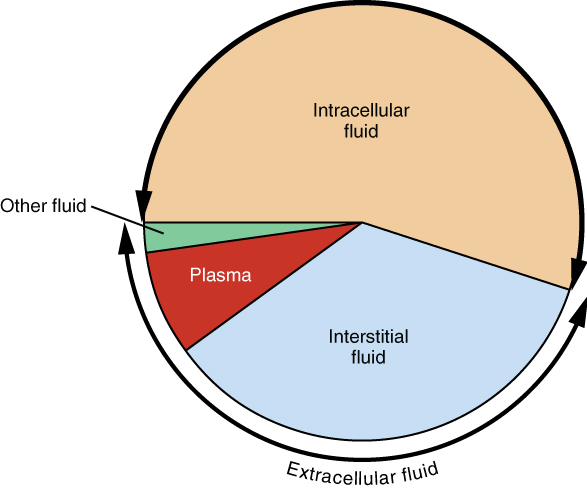
1. Intracellular Fluid: The intracellular fluid lies within cells and is the principal component of the cytosol/cytoplasm. The intracellular fluid makes up more than half, about 55%, of the total water in the human body, accounting for about 20 litres in an average adult human (Figure 4). This fluid volume tends to be very stable, because the amount of water in living cells is closely regulated. If the amount of water inside a cell falls to a value that is too low, the cytosol becomes too concentrated with solutes to carry on normal cellular activities; if too much water enters a cell, the cell may burst and be destroyed.
2. Extracellular Fluid: The extracellular fluid accounts for the remainder of the body’s water content. Approximately 16% of the extracellular fluid is found in plasma. Plasma travels through the body in blood vessels and transports a range of materials, including blood cells, proteins (including clotting factors and antibodies), electrolytes, nutrients, gases, and wastes. Gases, nutrients, and waste materials travel between capillaries and cells through the interstitial fluid. Interstitial fluid is the fluid surrounding living cells in every tissue, and accounts for nearly 80% of the extracellular fluid.Cells are separated from the interstitial fluid by a selectively permeable cell membrane that helps regulate the passage of materials between the interstitial fluid and the interior of the cell.
The body has other water-based extracellular fluid. These include the cerebrospinal fluid that bathes the brain and spinal cord, lymph, the synovial fluid in joints, the pleural fluid in the pleural cavities, the pericardial fluid in the cardiac sac, the peritoneal fluid in the peritoneal cavity, and the aqueous humor of the eye. Because these fluids are outside cells, these fluids are also considered components of the extracellular fluid compartment.
Composition of Body Fluids
The compositions of the two components of the extracellular fluid—plasma and interstitial fluid—are more similar to each other than either is to the intracellular fluid (Figure 5). Blood plasma has high concentrations of sodium, chloride, bicarbonate, and protein. The interstitial fluid has high concentrations of sodium, chloride, and bicarbonate, but a relatively lower concentration of protein. In contrast, the intracellular fluid has elevated amounts of potassium, phosphate, magnesium, and protein. Overall, the intracellular fluid contains high concentrations of potassium and phosphate (HPO42−), whereas both plasma and the extracellular fluid contain high concentrations of sodium and chloride.
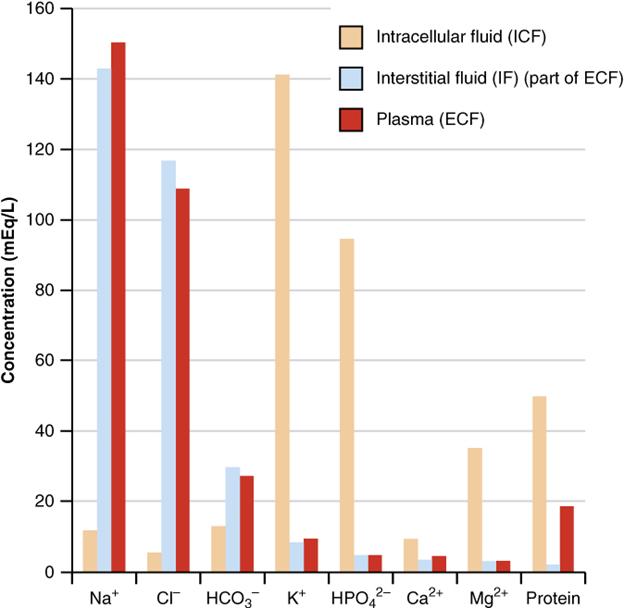
Body fluids are neutral in charge. Thus, cations, or positively charged ions, and anions, or negatively charged ions, are balanced in fluids. As seen in the previous graph, sodium (Na+) ions and chloride (Cl–) ions are concentrated in the extracellular fluid of the body, whereas potassium (K+) ions are concentrated inside cells. Although sodium and potassium can “leak” through “pores” into and out of cells, respectively, the high levels of potassium and low levels of sodium in the intracellular fluid are maintained by sodium-potassium pumps in the cell membranes. These pumps use the energy supplied by ATP to pump sodium out of the cell and potassium into the cell (Figure 6).
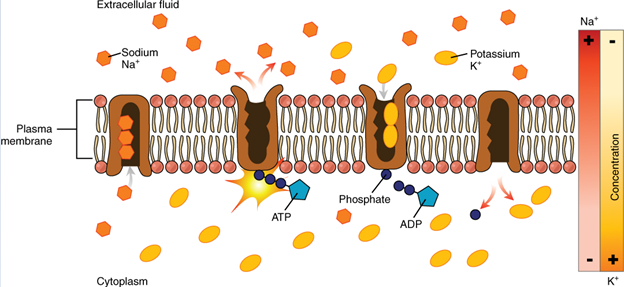
Roles of Electrolytes
The body contains a large variety of ions, or electrolytes, which perform a variety of functions. Some ions assist in the transmission of electrical impulses along cell membranes in neurons and muscles . Other ions help to stabilize protein structures in enzymes. Still others aid in releasing hormones from endocrine glands. All of the ions in plasma contribute to the osmotic balance that controls the movement of water between cells and their environment.
Electrolytes in living systems include sodium, potassium, chloride, bicarbonate, calcium, phosphate, magnesium, copper, zinc, iron, manganese, molybdenum, copper, and chromium. In terms of body functioning, six electrolytes are most important: sodium, potassium, chloride, bicarbonate, calcium, and phosphate.
These six ions aid in nerve excitability, endocrine secretion, membrane permeability, buffering body fluids, and controlling the movement of fluids between compartments. These ions enter the body through the digestive tract. More than 90 percent of the calcium and phosphate that enters the body is incorporated into bones and teeth, with bone serving as a mineral reserve for these ions. In the event that calcium and phosphate are needed for other functions, bone tissue can be broken down to supply the blood and other tissues with these minerals. Phosphate is a normal constituent of nucleic acids; hence, blood levels of phosphate will increase whenever nucleic acids are broken down.
Excretion of ions occurs mainly through the kidneys, with lesser amounts lost in sweat and in feces. Excessive sweating may cause a significant loss, especially of sodium and chloride. Severe vomiting or diarrhea will cause a loss of chloride and bicarbonate ions. Adjustments in respiratory and renal functions allow the body to regulate the levels of these ions in the extracellular fluid.
Part 3. Acid-Base Balance
Acids and bases, like salts, dissociate in water into electrolytes. Acids and bases can very much change the properties of the solutions in which they are dissolved.
Acids
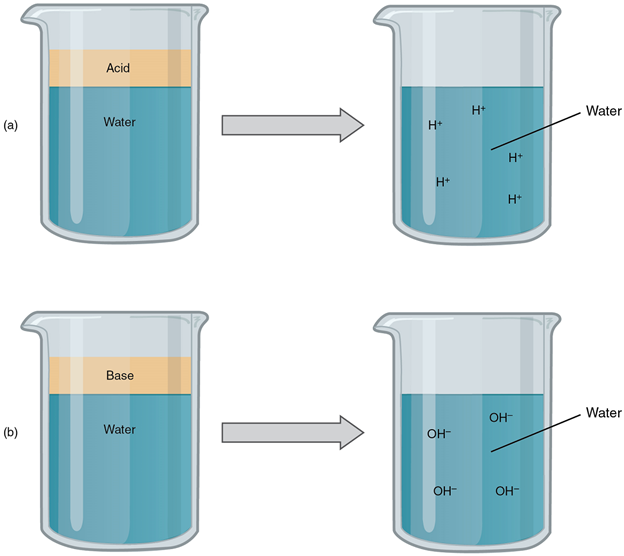
An acid is a substance that releases hydrogen ions (H+) in solution (Figure 7a). Because an atom of hydrogen has just one proton and one electron, a positively charged hydrogen ion is simply a proton. This solitary proton is highly likely to participate in chemical reactions. Strong acids are compounds that release all of their H+ in solution; that is, they ionize completely. Hydrochloric acid (HCl), which is released from cells in the lining of the stomach, is a strong acid because it releases all of its H+ in the stomach’s watery environment. This strong acid aids in digestion and kills ingested microbes. Weak acids do not ionize completely; that is, some of their hydrogen ions remain bonded within a compound in solution. An example of a weak acid is vinegar, or acetic acid; it is called acetate after it gives up a proton. Other common examples used in cellular metabolism include pyruvic acid, citric acid, and oxaloacetic acid which in water may release a proton to become pyruvate, citrate, and oxaloacetate respectively.
Bases
A base is a substance that releases hydroxyl ions (OH–) in solution, or one that accepts H+ already present in solution (Figure 7b). The hydroxyl ions (also known as hydroxide ions) or other basic substances combine with H+ present to form a water molecule, thereby removing H+ and reducing the solution’s acidity. Strong bases release most or all of their hydroxyl ions; weak bases release only some hydroxyl ions or absorb only a few H+.
The Concept of pH
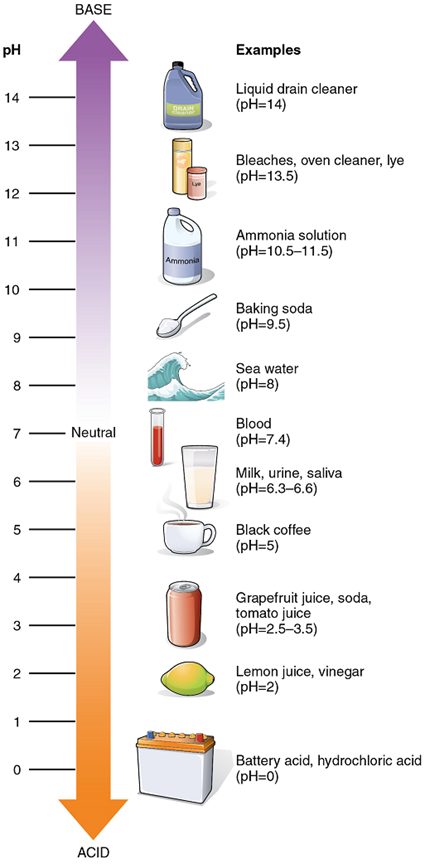
The relative acidity or alkalinity of a solution can be indicated by its pH. pH literally means the “potential of hydrogen”. It is a measure of the amount of hydrogen ions present per litre of a solution, expressed in grams. In technical terms, pH is the logarithm of the reciprocal of the hydrogen ion concentration of a solution.
As an example, a pH 4 solution has an H+ concentration that is ten times greater than that of a pH 5 solution. That is, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5. The concept of pH will begin to make more sense when you study the pH scale (Figure 8). The scale consists of a series of increments ranging from 0 to 14. A solution with a pH of 7 is considered neutral—neither acidic nor basic. Pure water has a pH of 7. The lower the number below 7, the more acidic the solution, or the greater the concentration of H+. The concentration of hydrogen ions at each pH value is 10 times different than the next pH. The higher the number above 7, the more basic (alkaline) the solution, or the lower the concentration of H+.
Buffers
The pH of human blood normally ranges from 7.35 to 7.45. At this slightly basic pH, blood can reduce the acidity resulting from the carbon dioxide (CO2) constantly being released into the bloodstream by the trillions of cells in the body. Homeostatic mechanisms (along with exhaling CO2 while breathing) normally keep the pH of blood within this narrow range. This is critical, because fluctuations—either too acidic or too alkaline—can lead to life-threatening disorders.
All cells of the body depend on homeostatic regulation of acid–base balance at a very narrow range of pH between 7.35 to 7.45. The body therefore has several mechanisms for this regulation, involving breathing, the excretion of chemicals in urine, and the internal release of chemicals collectively called buffers into body fluids. A buffer is a solution of a weak acid and its conjugate base. A buffer can resist sudden changes in the acidity and alkalinity of the body fluids. For example, if there is even a slight decrease below 7.35 in the pH of a bodily fluid, the buffer in the fluid—in this case, acting as a weak base—will bind the excess hydrogen ions.
In contrast, if pH rises above 7.45, the buffer will act as a weak acid and contribute hydrogen ions.
Acid-Base Balance: Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. Acid-base balance is measured using the pH scale (Figure 8). A variety of buffering systems permits blood and other bodily fluids to maintain a narrow pH range, even in the face of perturbations. A buffer is a chemical system that minimizes change in hydrogen ion concentration.
Buffer Systems in the Body: The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical buffers in the blood to resist changes in the pH. The respiratory tract can adjust the blood pH upward in minutes by exhaling CO2 from the body. The renal system can also adjust blood pH through the excretion of hydrogen ions (H+) and the conservation of bicarbonate, but this process takes hours to days to have an effect.
The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The kidneys help control acid-base balance by excreting hydrogen ions and generating bicarbonate that helps maintain blood plasma pH within a normal range.
Protein Buffers in Blood Plasma and Cells: Protein buffer systems work predominantly inside cells. Nearly all proteins can function as buffers. Proteins are made up of amino acids, which contain positively charged amino groups and negatively charged carboxyl groups. The charged regions of free amino acids can bind hydrogen and hydroxyl ions, and thus function as buffers. Buffering by proteins accounts for two-thirds of the buffering power of the blood and most of the buffering within cells.
Phosphate Buffer: Phosphates are found in the blood in two forms: sodium dihydrogen phosphate (NaH2PO4), which is a weak acid, and sodium monohydrogen phosphate (Na2HPO4), which is a weak base. When Na2HPO4 comes into contact with a strong acid, such as HCl, the base reacts with the hydrogen ion released by the HCl to form the weak acid NaH2PO4 and sodium chloride (NaCl). When NaH2PO4 (the weak acid) comes into contact with a strong base, such as sodium hydroxide (NaOH), the weak acid releases H+ ions which bind to the OH– ions released by the base to produce water.
HCl + Na2HPO4→NaH2PO4 + NaCl
(strong acid) + (weak base) → (weak acid) + (a salt)
NaOH + NaH2PO4→Na2HPO4 + H2O
(strong base) + (weak acid) → (weak base) + (water)
Bicarbonate-Carbonic Acid Buffer: The bicarbonate-carbonic acid buffer works in a fashion similar to phosphate buffers. When sodium bicarbonate (NaHCO3), comes into contact with a strong acid, such as HCl, carbonic acid (H2CO3), which is a weak acid, and NaCl are formed. When carbonic acid comes into contact with a strong base, such as NaOH, bicarbonate and water are formed.
NaHCO3 + HCl → H2CO3 + NaCl
(sodium bicarbonate) + (strong acid) → (weak acid) + (a salt)
H2CO3 + NaOH→ NaHCO3 + H2O
(weak acid) + (strong base) → (sodium bicarbonate) + (water)
As with the phosphate buffer, a weak acid or weak base captures the free ions, and a significant change in pH is prevented. Bicarbonate ions and carbonic acid are present in the blood in a 20:1 ratio if the blood pH is within the normal range. With 20 times more bicarbonate than carbonic acid, this capture system is most efficient at buffering changes that would make the blood more acidic. This is useful because most of the body’s metabolic wastes, such as lactic acid and ketone bodies, are acids. Carbonic acid levels in the blood are controlled by the expiration of CO2 through the lungs. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are conserved and passed back into the blood. However, the bicarbonate buffer is the primary buffering system of the interstitial fluid surrounding the cells in tissues throughout the body.
Respiratory Regulation of Acid-Base Balance: The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (Figure 9). CO2 in the blood readily reacts with water to form carbonic acid, and the levels of CO2 and carbonic acid in the blood are in equilibrium. When the CO2 level in the blood rises (as it does when you hold your breath), the excess CO2 reacts with water to form additional carbonic acid, lowering blood pH. Increasing the rate and/or depth of respiration (which you might feel the “urge” to do after holding your breath) allows you to exhale more CO2. The loss of CO2 from the body reduces blood levels of carbonic acid and thereby adjusts the pH upward, toward normal levels. This process also works in the opposite direction, excessive deep and rapid breathing (as in hyperventilation) rids the blood of CO2 and reduces the level of carbonic acid, making the blood too alkaline.
Renal Regulation of Acid-Base Balance: The renal regulation of the body’s acid-base balance addresses the metabolic component of the buffering system. Whereas the respiratory system (together with breathing centres in the brain) controls the blood levels of carbonic acid by controlling the exhalation of CO2, the renal system controls the blood levels of bicarbonate.
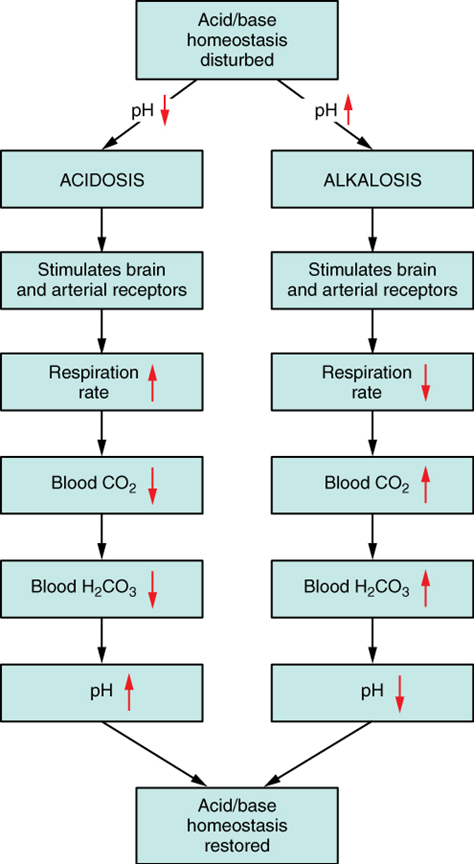
A decrease of blood bicarbonate can result by certain diuretics or from excessive bicarbonate loss due to diarrhea. Low bicarbonate blood levels can also occur as a result of elevated levels of ketone bodies (common in unmanaged diabetes mellitus), which bind bicarbonate hence, lowering their concentration in the blood.
Practice Questions
Part 1. Biological importance of water
Part 2. Fluid compartments in the human body
Part 3. Acid-Base Balance
Thick, lubricating fluid that fills the interior of a synovial joint.
Substance that acts as a lubricant for the visceral and parietal layers of the pleura during the movement of breathing.
Scatter or break up.
Dipole-dipole bond in which a hydrogen atom covalently bonded to an electronegative atom is weakly attracted to a second electronegative atom.
A substance composed of two or more different elements joined by chemical bonds.
In chemistry, a homogeneous liquid mixture in which a solute is dissolved into molecules within a solvent.
Component of a solution, the substance that dissolves the solute.
Component of a solution, the substance dissolved in a solvent.
Condition in which solute molecules are distributed equally in a solution.
A functional group, OH, present in many organic compounds including alcohols.
Chemical reaction in which a molecule water is split into H and OPH, thereby breaking a bond and severing a compound.
Fluid inside cells.
An extracellular fluid, the fluid component of blood.
Extracellular fluid in the small spaces between cells not contained within blood vessels.
Clear, semi-fluid medium of the cytoplasm, made up mostly of water.
Internal material between the cell membrane and nucleus of a cell, mainly consisting of a water-based fluid called cytosol, within which are all the other organelles and cellular solute and suspended materials.
(Also, immunoglobulin) antigen-specific protein secreted by plasma cells.
A solution containing ions; sometimes referring to ions themselves.
Smallest of the blood vessels where physical exchange occurs between the blood and tissue cells surrounded by interstitial fluid.
Feature of any barrier that allows certain substances to cross but excludes others.
Circulatory medium within the CNS that is produced by ependymal cells in the choroid plexus filtering the blood.
Fluid contained within the lymphatic system, consisting of interstitial fluid, leukocytes (white blood cells), proteins (including antibodies) and fats.
The space between the visceral and parietal pleurae.
Fluid found in the pericardium.

