Unit 17: Neuronal Signalling
Unit Outline
Part 2: Ion Channels and the Resting Membrane Potential
Part 3: Generation of an Action Potential
Part 4: Propagation of Action Potentials
Practice Questions
Learning Outcomes
At the end of this unit, you should be able to:
I. Describe the resting membrane potential of a neuron and explain how it is maintained.
II. Explain how graded and action potentials are generated in neurons.
III. Explain how neuronal action potentials travel down the axon.
IV. Explain the process of neurotransmission, and name and explain the functions of various different neurotransmitters.
Part 1: Neuronal Signalling
Having looked at the components of nervous tissue, and the basic anatomy of the nervous system, next comes an understanding of how nervous tissue is capable of communicating within the nervous system. Before getting to the nuts and bolts of how this works, an illustration of how the components come together will be helpful (summarized in Figure 1).
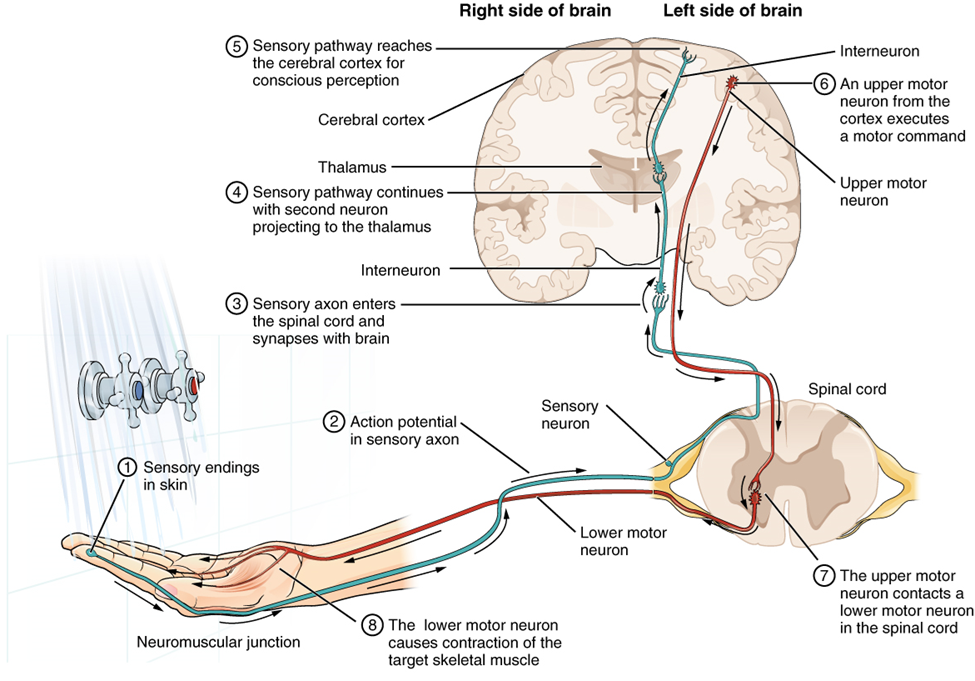
Imagine you are about to take a shower. You have turned on the faucet to start the water as you prepare to get in the shower. After a few minutes, you expect the water to be a temperature that will be comfortable to enter. So you put your hand out into the spray of water. What happens next depends on how your nervous system interacts with the stimulus of the water temperature and what you do in response to that stimulus.
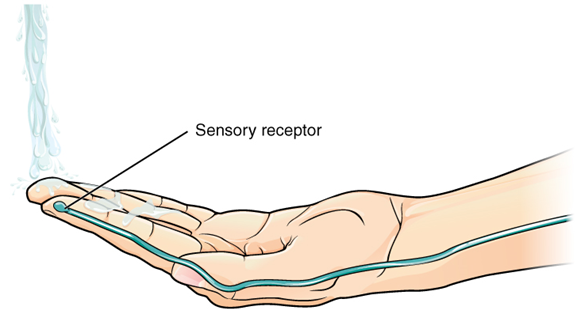
Found in the skin of your fingers or toes is a type of sensory receptor that is sensitive to temperature, called a thermoreceptor. When you place your hand under the shower (Figure 2), the cell membrane of the thermoreceptors changes its electrical state (voltage). The amount of change is dependent on the strength of the stimulus (how hot the water is). This is called a graded potential. If the stimulus is strong, the voltage of the cell membrane will change enough to generate an electrical signal that will travel down the axon.
The voltage at which such a signal is generated is called the threshold, and the resulting electrical signal is called an action potential. In this example, the action potential travels—a process known as propagation—along the axon from the axon hillock to the axon terminals and into the synaptic end bulbs. When this signal reaches the end bulbs, it causes the release of a signaling molecule called a neurotransmitter.
The neurotransmitter diffuses across the short distance of the synapse and binds to a receptor protein of the target neuron. When the molecular signal binds to the receptor, the cell membrane of the target neuron changes its electrical state and a new graded potential begins. If that graded potential is strong enough to reach threshold, the second neuron generates an action potential at its axon hillock. The target of this neuron is another neuron in the thalamus of the brain, the part of the central nervous system that acts as a relay for sensory information. At another synapse, neurotransmitter is released and binds to its receptor. The thalamus then sends the sensory information to the cerebral cortex, the outermost layer of grey matter in the brain, where conscious perception of that water temperature begins. Within the cerebral cortex, information is processed among many neurons, integrating the stimulus of the water temperature with other sensory stimuli, with your emotional state (you just aren’t ready to wake up; the bed is calling to you), memories (perhaps of the lab notes you have to study before a quiz). Finally, a plan is developed about what to do, whether that is to turn the temperature up, turn the whole shower off and go back to bed, or step into the shower. To do any of these things, the cerebral cortex has to send a command out to your body to move muscles (Figure 3).
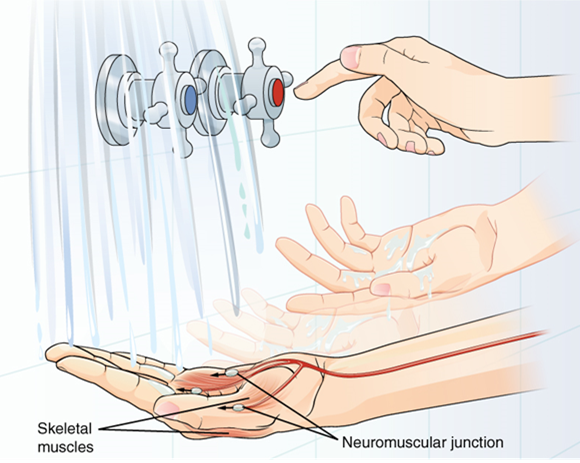
A region of the cortex is specialized for sending signals down to the spinal cord for movement. The upper motor neuron is in this region, called the primary motor cortex, which has an axon that extends all the way down the spinal cord. At the level of the spinal cord at which this axon makes a synapse, a graded potential occurs in the cell membrane of a lower motor neuron. This second motor neuron is responsible for causing muscle fibres to contract. In the manner described in the chapter on muscle tissue, an action potential travels along the motor neuron axon into the periphery. The axon terminates on muscle fibers at the neuromuscular junction. Acetylcholine is released at this specialized synapse, which causes the muscle action potential to begin, following a large potential known as an end plate potential. When the lower motor neuron excites the muscle fiber, it contracts. All of this occurs in a fraction of a second, but this story is the basis of how the nervous system functions.
Part 2: Ion Channels and the Resting Membrane Potential
The functions of the nervous system—sensation, integration, and response—depend on the functions of the neurons underlying these pathways. To understand how neurons are able to communicate, it is necessary to describe the role of an excitable membrane in generating these signals. The basis of this communication is the action potential, which demonstrates how changes in the membrane can constitute a signal. (The way these signals work in more variable circumstances involves graded potentials.)
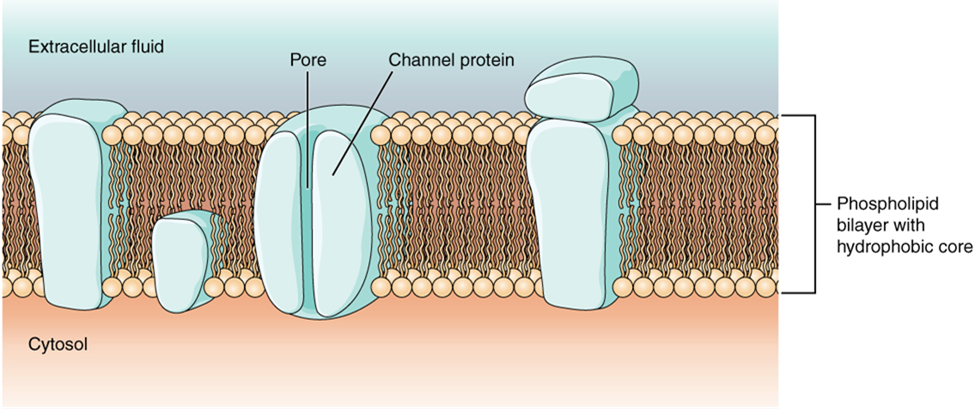
Most cells in the body make use of charged particles, ions, to build up a charge across the cell membrane. Cells make use of the cell membrane to regulate ion movement between the extracellular fluid and cytosol. As you learned in the chapter on cells, the cell membrane is primarily responsible for regulating what can cross the membrane and what stays on only one side. The cell membrane is a phospholipid bilayer, so only substances that can pass directly through the hydrophobic core can diffuse through unaided. Charged particles, which are hydrophilic by definition, cannot pass through the cell membrane without assistance (Figure 4). Transmembrane proteins, specifically channel proteins, make this possible. Several passive ion channels, as well as active transport pumps, are necessary to generate a transmembrane potential and an action potential. Ion channels are pores that allow specific charged particles to cross the membrane in response to an existing concentration gradient.
Of special interest is the carrier protein referred to as the sodium/potassium pump that moves sodium ions (Na+) out of a cell and potassium ions (K+) into a cell, thus regulating ion concentration on both sides of the cell membrane. The sodium/potassium pump requires energy in the form of adenosine triphosphate (ATP), so it is also referred to as an ATPase. As was explained in the cell chapter, the concentration of Na+ is higher outside the cell than inside, and the concentration of K+ is higher inside the cell than outside. That means that this pump is moving the ions against the concentration gradients for sodium and potassium, which is why it requires energy. In fact, the pump basically maintains those concentration gradients.
Ion channels do not always freely allow ions to diffuse across the membrane. Some are opened by certain events, meaning the channels are gated.
A ligand-gated channel opens because a signaling molecule, a ligand, binds to the extracellular region of the channel. This type of channel is also known as an ionotropic receptor because when the ligand, known as a neurotransmitter in the nervous system, binds to the protein, ions cross the membrane changing its charge (Figure 5).
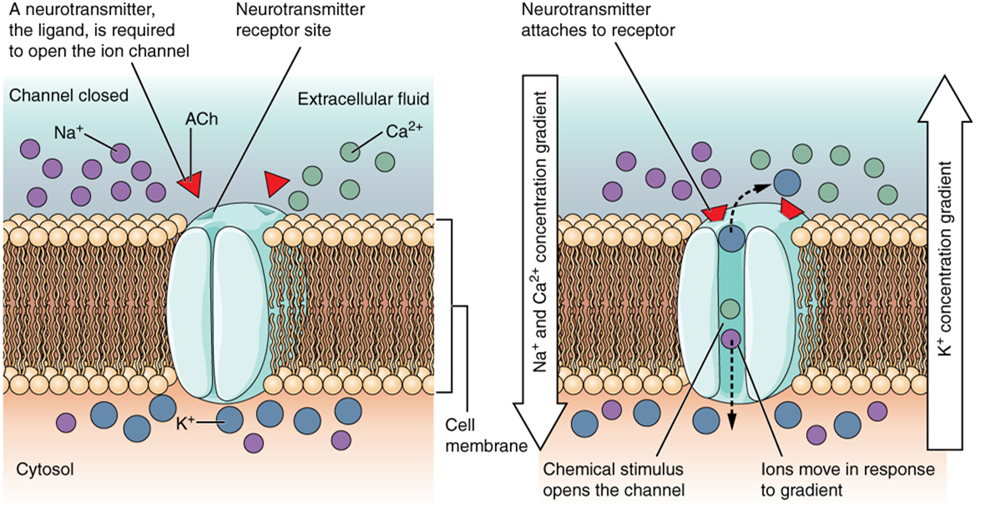
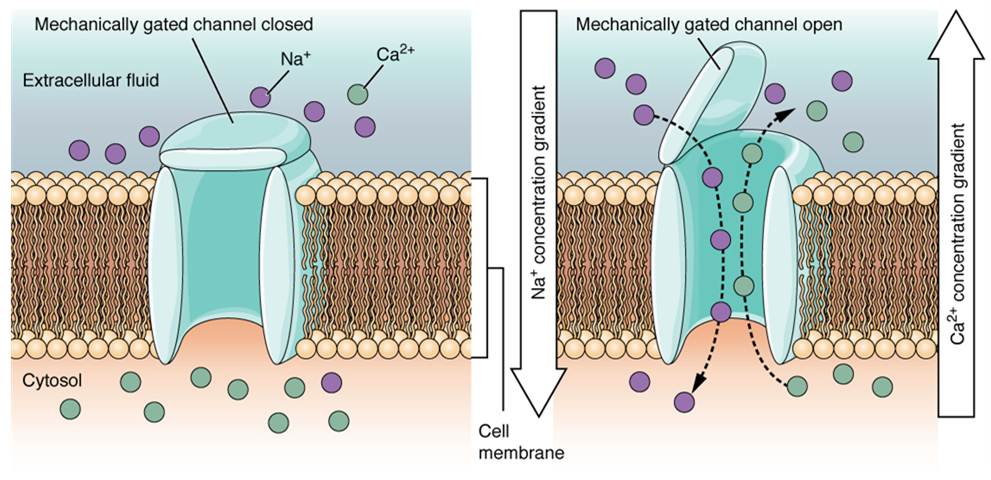
A mechanically gated channel (or mechanoceptor) opens because of a physical distortion of the cell membrane. Many channels associated with the sense of touch (somatosensation) are mechanically gated. For example, as pressure is applied to the skin, these channels open and allow ions to enter the cell. Similar to this type of channel would be the channel that opens on the basis of temperature changes, (a thermoreceptor) as in testing the water in the shower (Figure 6).
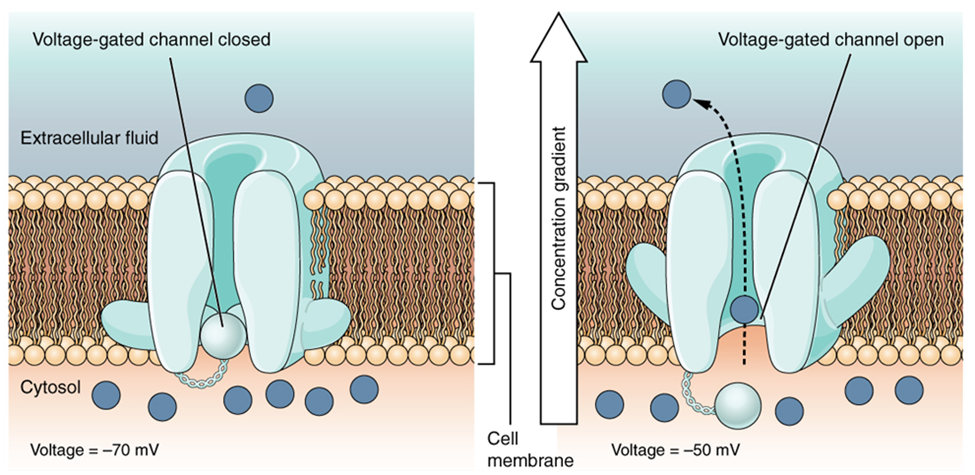
A voltage-gated channel is a channel that responds to changes in the electrical properties of the membrane in which it is embedded. Normally, the inner portion of the membrane is at a negative voltage. When that voltage becomes less negative, the channel begins to allow ions to cross the membrane (Figure 7).
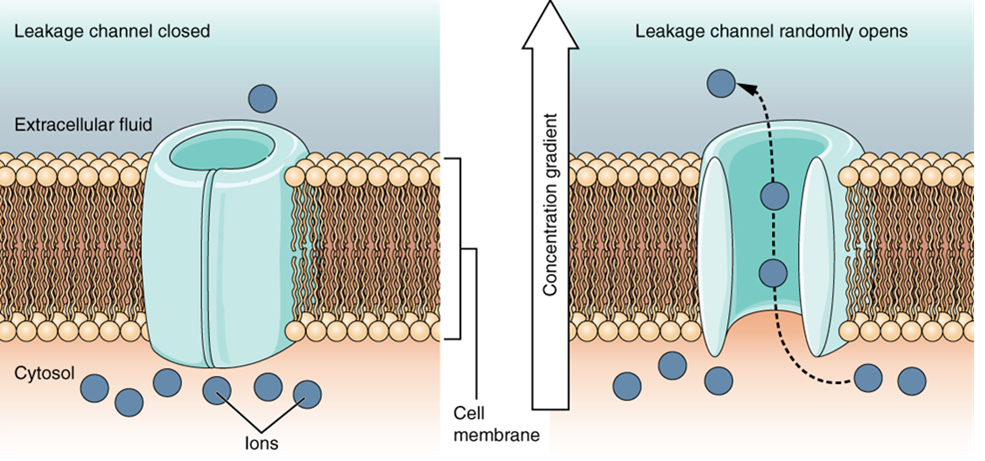
A leakage channel is randomly gated, meaning that it opens and closes at random, hence the reference to leaking. There is no actual event that opens the channel; instead, it has an intrinsic rate of switching between the open and closed states. Leakage channels contribute to the resting transmembrane voltage of the excitable membrane (Figure 8).
The electrical state of the cell membrane can have several variations. These are all variations in the membrane potential. A potential is a distribution of charge across the cell membrane, measured in millivolts (mV). The standard is to compare the inside of the cell relative to the outside, so the membrane potential is a value representing the charge on the intracellular side of the membrane based on the outside being zero, relatively speaking (Figure 9).
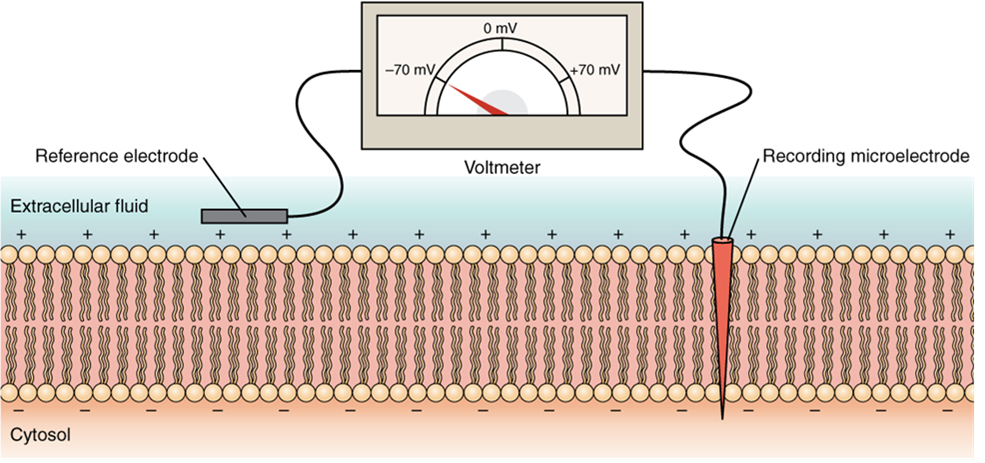
The concentration of ions in extracellular and intracellular fluids is largely balanced, with a net neutral charge. However, a slight difference in charge occurs right at the membrane surface, both internally and externally. It is the difference in this very limited region that has all the power in neurons (and muscle cells) to generate electrical signals, including action potentials.
Before these electrical signals can be described, the resting state of the membrane must be explained. When the cell is at rest, and the ion channels are closed (except for leakage channels which randomly open), ions are distributed across the membrane in a very predictable way. The concentration of Na+ outside the cell is 10 times greater than the concentration inside. Also, the concentration of K+ inside the cell is greater than outside. The cytosol contains a high concentration of anions, in the form of phosphate ions and negatively charged proteins. Large anions are a component of the inner cell membrane, including specialized phospholipids and proteins associated with the inner leaflet of the membrane (leaflet is a term used for one side of the lipid bilayer membrane). The negative charge is localized in the large anions.
With the ions distributed across the membrane at these concentrations, the difference in charge is measured at -70 mV, the value described as the resting membrane potential. The exact value measured for the resting membrane potential varies between cells, but -70 mV is the most commonly recorded value. This voltage would actually be much lower except for the contributions of some important proteins in the membrane. Leakage channels K+ channels allow K+ to slowly move out of the cells. To a much lesser extent, leakage Na+ channels allow Na+ to slowly move into the cell. The constant activity of the Na+/K+ pump maintains the ion gradients. This may appear to be a waste of energy, but each has a role in maintaining the membrane potential.
Part 3: Generation of an Action Potential
Resting membrane potential describes the steady state of the cell, which is a dynamic process that is balanced by ion leakage and ion pumping. Without any outside influence, it will not change. To get an electrical signal started, the membrane potential has to change.
As mentioned above, local changes in the membrane potential are called graded potentials and these are usually associated with the dendrites of a neuron. The amount of change in the membrane potential is determined by the size of the stimulus that causes it. In the example of testing the temperature of the shower, slightly warm water would only initiate a small change in a thermoreceptor, whereas hot water would cause a large amount of change in the membrane potential.
Graded potentials can be of two sorts, either they are depolarizing or hyperpolarizing (Figure 10). For a membrane at the resting potential, a graded potential represents a change in that voltage either above -70 mV or below -70 mV. Depolarizing graded potentials are often the result of Na+ or Ca2+ entering the cell. Both of these ions have higher concentrations outside the cell than inside; because they have a positive charge, they will move into the cell causing it to become less negative relative to the outside. Hyperpolarizing graded potentials can be caused by K+ leaving the cell or Cl– entering the cell. If a positive charge moves out of a cell, the cell becomes more negative; if a negative charge enters the cell, the same thing happens.
Action potentials, on the other hand are describes as “all or none” because they either occur and change the membrane voltage a given amount (not graded), or they do not occur at all. Action potentials start at a neuron’s axon hillock, or near the motor end plate of a skeletal muscle, with a channel opening for Na+ in the membrane. Because the concentration of Na+ is higher outside the cell than inside the cell by a factor of 10, ions will rush into the cell that are driven largely by the concentration gradient. Because sodium is a positively charged ion, it will change the relative voltage immediately inside the cell relative to immediately outside. The resting potential is the state of the membrane at a voltage of -70 mV, so the sodium cation entering the cell will cause it to become less negative. This is known as depolarization, meaning the membrane potential moves toward zero.
The concentration gradient for Na+ is so strong that it will continue to enter the cell even after the membrane potential has become zero, so that the voltage immediately around the pore begins to become positive. The electrical gradient also plays a role, as negative proteins below the membrane attract the sodium ion. The membrane potential will reach +30 mV by the time sodium has entered the cell.
As the membrane potential reaches +30 mV, the sodium channels become inactivated, preventing further entry of sodium ions, and other voltage-gated channels open in the membrane. These channels are specific for the potassium ion. A concentration gradient acts on K+, as well. As K+ starts to leave the cell, taking a positive charge with it, the membrane potential begins to move back toward its resting voltage. This is called repolarization, meaning that the membrane voltage moves back toward the -70 mV value of the resting membrane potential.
Repolarization returns the membrane potential to the -70 mV value that indicates the resting potential, but it actually overshoots that value. Potassium ions reach equilibrium when the membrane voltage is below -70 mV, so a period of hyperpolarization occurs while the K+ channels are open. Those K+ channels are slightly delayed in closing, accounting for this short overshoot.
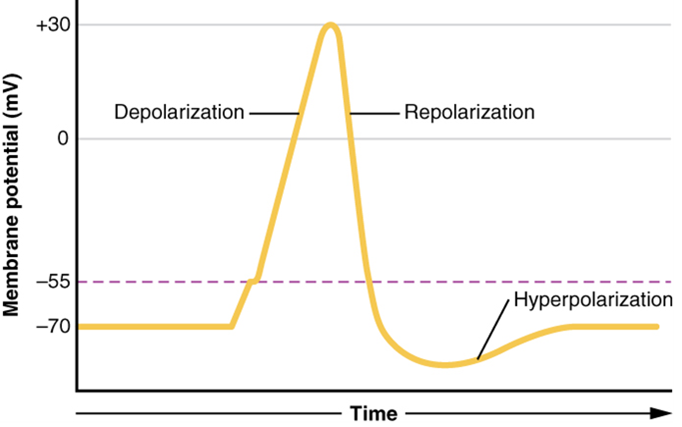
What has been described here is the action potential, which is presented as a graph of voltage over time (Figure 11). It is the electrical signal that nervous tissue generates for communication. The change in the membrane voltage from -70 mV at rest to +30 mV at the end of depolarization is a 100-mV change. That can also be written as a 0.1-V change. To put that value in perspective, think about a battery. An AA battery that you might find in a television remote has a voltage of 1.5 V, or a 9-V battery (the rectangular battery with two posts on one end) is, obviously, 9 V. The change seen in the action potential is one or two orders of magnitude less than the charge in these batteries. In fact, the membrane potential can be described as a battery. A charge is stored across the membrane that can be released under the correct conditions. A battery in your remote has stored a charge that is “released” when you push a button.
The question is, now, what initiates the action potential? The description above conveniently glosses over that point. But it is vital to understanding what is happening. The membrane potential will stay at the resting voltage until something changes. The description above just says that a Na+ channel opens. Now, to say “a channel opens” does not mean that one individual transmembrane protein changes. Instead, it means that one kind of channel opens. There are a few different types of channels that allow Na+ to cross the membrane. A ligand-gated Na+ channel will open when a neurotransmitter binds to it and a mechanically gated Na+ channel will open when a physical stimulus affects a sensory receptor (like pressure applied to the skin compresses a touch receptor). Whether it is a neurotransmitter binding to its receptor protein or a sensory stimulus activating a sensory receptor cell, some stimulus gets the process started. Sodium starts to enter the cell and the membrane becomes less negative.
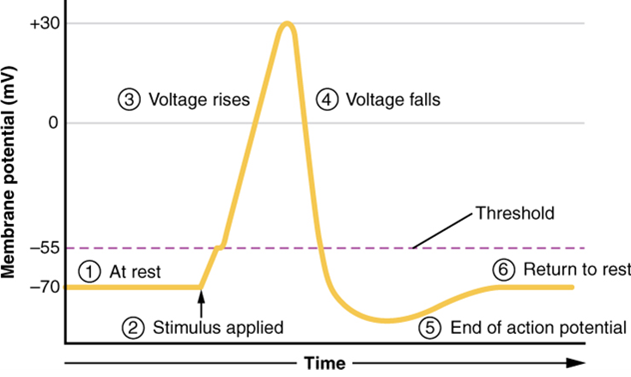
A third type of channel that is an important part of depolarization in the action potential is the voltage-gated Na+ channel. The channels that start depolarizing the membrane because of a stimulus help the cell to depolarize from -70 mV to -55 mV. Once the membrane reaches that voltage, the voltage-gated Na+ channels open. This is what is known as the threshold. Any depolarization that does not change the membrane potential to -55 mV or higher will not reach threshold and thus will not result in an action potential. Also, any stimulus that depolarizes the membrane to -55 mV or beyond will cause a large number of channels to open and an action potential will be initiated.
Because of the threshold, the action potential can be likened to a digital event—it either happens or it does not. If the threshold is not reached, then no action potential occurs. If depolarization reaches -55 mV, then the action potential continues and runs all the way to +30 mV, at which K+ causes repolarization, including the hyperpolarizing overshoot (also called an undershoot, since the voltage is under that of the resting membrane potential). Also, those changes are the same for every action potential, which means that once the threshold is reached, the exact same thing happens. A stronger stimulus, which might depolarize the membrane well past threshold, will not make a “bigger” action potential. Action potentials are “all or none.” Either the membrane reaches the threshold and everything occurs as described above, or the membrane does not reach the threshold and nothing else happens. All action potentials peak at the same voltage (+30 mV), so one action potential is not bigger than another. Stronger stimuli will initiate multiple action potentials more quickly, but the individual signals are not bigger. Thus, for example, you will not feel a greater sensation of pain, or have a stronger muscle contraction, because of the size of the action potential because they are not different sizes.
As we have seen, the depolarization and repolarization of an action potential are dependent on two types of channels (the voltage-gated Na+ channel and the voltage-gated K+ channel). The voltage-gated Na+ channel actually has two gates. One is the activation gate, which opens when the membrane potential crosses -55 mV. The other gate is the inactivation gate, which closes after a specific period of time at a depolarized voltage—on the order of a fraction of a millisecond. When a cell is at rest, the activation gate is closed and the inactivation gate is open. However, when the threshold is reached, the activation gate opens, allowing Na+ to rush into the cell. Timed with the peak of depolarization, the inactivation gate closes. During repolarization, no more sodium can enter the cell. When the membrane potential passes -55 mV again, the activation gate closes. After that, the inactivation gate re-opens, making the channel ready to start the whole process over again.
The voltage-gated K+ channel has only one gate, which is sensitive to a membrane voltage of -50 mV. However, it does not open as quickly as the voltage-gated Na+ channel does. It might take a fraction of a millisecond for the channel to open once that voltage has been reached. The timing of this coincides exactly with when the Na+ flow peaks, so voltage-gated K+ channels open just as the voltage-gated Na+ channels are being inactivated. As the membrane potential repolarizes and the voltage passes -50 mV again, the channel closes—again, with a little delay. Potassium continues to leave the cell for a short while and the membrane potential becomes more negative, resulting in the hyperpolarizing overshoot. Then the channel closes again and the membrane can return to the resting potential because of the ongoing activity of the non-gated channels and the Na+/K+ pump. All of this takes place within approximately 2 milliseconds (Figure 12). While an action potential is in progress, another one cannot be initiated. That effect is referred to as the refractory period.
Part 4: Propagation of Action Potentials
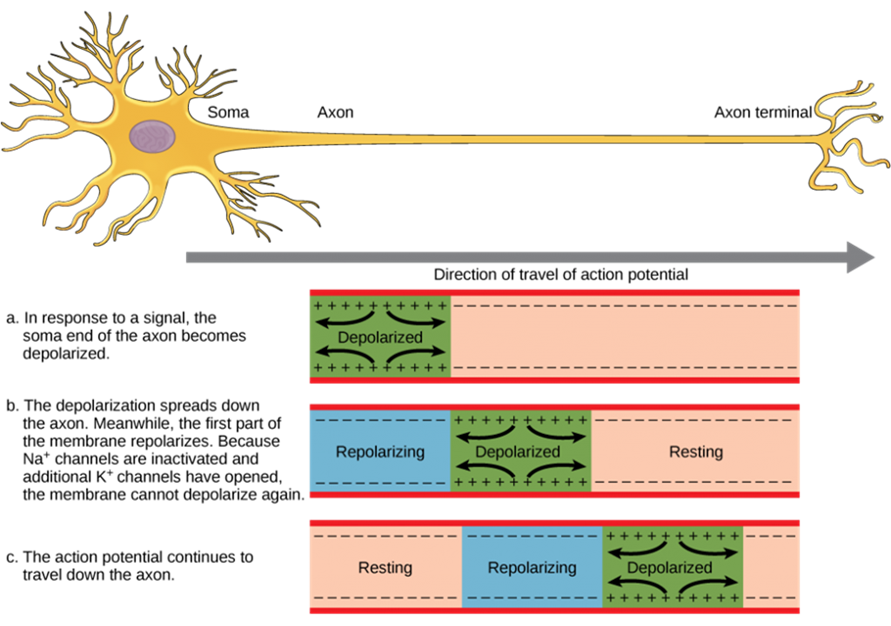
The action potential is initiated at the beginning of the axon, at what is called the initial segment. There is a high density of voltage-gated Na+ channels so that rapid depolarization can take place here. Going down the length of the axon, the action potential is propagated because more voltage-gated Na+ channels are opened as the depolarization spreads. This spreading occurs because Na+ enters through the channel and moves along the inside of the cell membrane. As the Na+ moves, or flows, a short distance along the cell membrane, its positive charge depolarizes a little more of the cell membrane. As that depolarization spreads, new voltage-gated Na+ channels open and more ions rush into the cell, spreading the depolarization a little farther (Figure 13).
Because voltage-gated Na+ channels are inactivated at the peak of the depolarization, they cannot be opened again for a brief time—the absolute refractory period. Because of this, depolarization spreading back toward previously opened channels has no effect. The action potential must propagate toward the axon terminals; as a result, the polarity of the neuron is maintained, as mentioned above. While the depolarization spreads toward the axon terminals, K+ channels open up in the previously depolarized segments, restoring them to resting membrane voltage, thus resetting them for another action potential to pass down the axon.
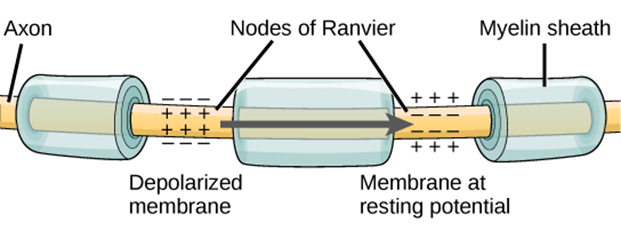
Propagation, as described above, applies to unmyelinated axons. When myelination is present, the action potential propagates differently (Figure 14). Sodium ions that enter the cell at the initial segment start to spread along the length of the axon segment, but there are no voltage-gated Na+ channels until the first node of Ranvier. Because there is not constant opening of these channels along the axon segment, the depolarization spreads at a much faster speed. The distance between nodes (1-3 mm) means that the membrane is still sufficiently depolarized (above threshold) by the time it reaches the next node. As Na+ spreads along the inside of the membrane of the axon segment, the charge starts to dissipate, but the myelin “insulation” keeps it from dissipating too quickly. If the node were farther down the axon, that depolarization would may have fallen off too much for voltage-gated Na+ channels to be activated at the next node of Ranvier.
Propagation along an unmyelinated axon is referred to as continuous conduction; along the length of a myelinated axon, it is saltatory conduction. Continuous conduction is slow because there are always voltage-gated Na+ channels opening, and more and more Na+ is rushing into the cell. Saltatory conduction is faster because the action potential basically jumps from one node to the next (saltare = “to leap”), and the new influx of Na+ renews the depolarized membrane. Along with the myelination of the axon, the diameter of the axon can influence the speed of conduction. Much as water runs faster in a wide river than in a narrow creek, Na+-based depolarization spreads faster down a wide axon than down a narrow one. This concept is known as resistance and is generally true for electrical wires or plumbing, just as it is true for axons, although the specific conditions are different at the scales of electrons or ions versus water in a river.
Part 5: Neurotransmission
The electrical changes taking place within a neuron, as described in the previous section, are similar to a light switch being turned on. A stimulus starts the depolarization, but the action potential runs on its own once a threshold has been reached. The question is now, “What flips the light switch on?” Temporary changes to the cell membrane voltage can result from neurons receiving information from the environment, or from the action of one neuron on another. These special types of potentials influence a neuron and determine whether an action potential will occur or not. Many of these transient signals originate at the synapse, the connection between electrically active cells, and then travel to the axon hillock as a graded potential.
There are two types of synapses: chemical synapses and electrical synapses. In a chemical synapse, a chemical signal—namely, a neurotransmitter—is released from one cell and it affects the other cell. In an electrical synapse, there is a direct connection between the two cells so that ions can pass directly from one cell to the next. If one cell is depolarized in an electrical synapse, the joined cell also depolarizes because the ions pass between the cells. Chemical synapses involve the transmission of chemical information from one cell to the next. This section will concentrate on the chemical type of synapse.
An example of a chemical synapse is the neuromuscular junction described in the chapter on muscle tissue. In the nervous system, there are many more synapses that are essentially the same as the neuromuscular junction. All synapses have common characteristics, which can be summarized in this list:
- presynaptic element
- neurotransmitter (packaged in vesicles)
- synaptic cleft
- receptor proteins
- postsynaptic element
- neurotransmitter elimination or re-uptake
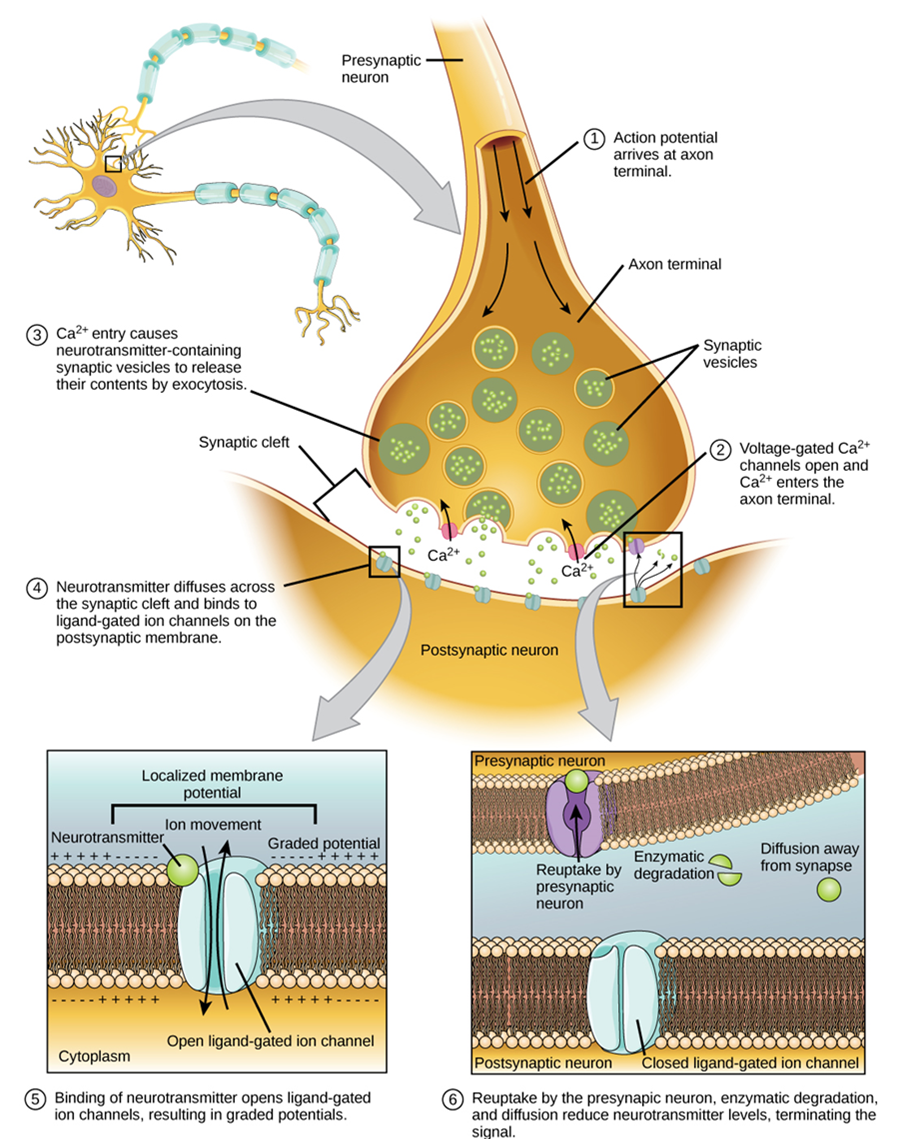
Synaptic transmission (or neurotransmission) takes place through the following steps (Figure 15):
- An action potential reaches the axon terminal.
- The change in voltage causes voltage-gated Ca2+ channels in the membrane of the synaptic end bulb to open.
- The concentration of Ca2+ increases inside the end bulb, and Ca2+ ions associate with proteins in the outer surface of neurotransmitter vesicles facilitating the merging of the vesicle with the presynaptic membrane. The neurotransmitter is then released through exocytosis into the small gap between the cells, known as the synaptic cleft.
- Once in the synaptic cleft, the neurotransmitter diffuses the short distance to the postsynaptic membrane and can interact with neurotransmitter receptors. Receptors are specific for the neurotransmitter, and the two fit together like a key and lock. One neurotransmitter binds to its receptor and will not bind to receptors for other neurotransmitters, making the binding a specific chemical event.
- The interaction of the neurotransmitter with the receptor can result in depolarization or hyperpolarization of the postsynaptic cell membrane, leading to excitation of the postsynaptic cell (and possibly the generation of a new action potential) or inhibition, respectively.
- The neurotransmitter is removed from the synaptic cleft by diffusion, due to the action of enzymes that break it down chemically or by transporters in the presynaptic cell membrane.
Neurotransmitter Systems
There are several systems of neurotransmitters that are found at various synapses in the nervous system (Figure 16). Some important examples are listed below.
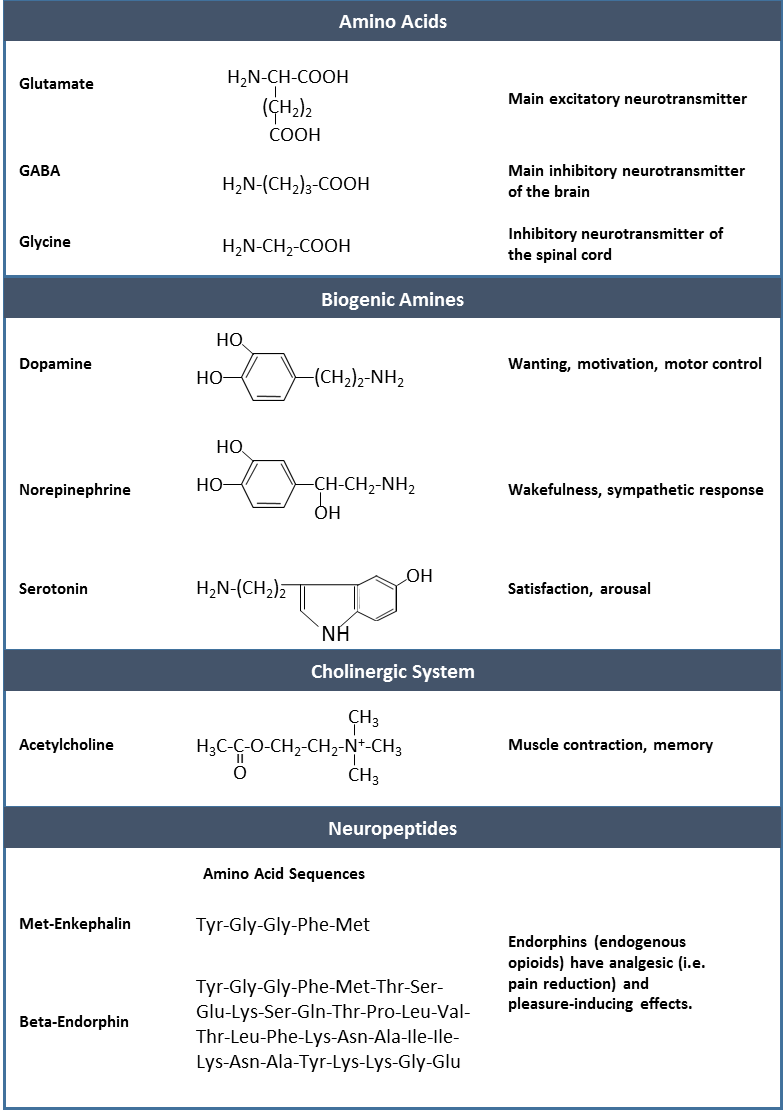
- Amino acids: This includes glutamate (Glu), GABA (gamma-aminobutyric acid, a derivative of glutamate), and glycine (Gly).
- Biogenic amines: This is a group of neurotransmitters that are enzymatically made from amino acids. For example, the neurotransmitter serotonin is made from tryptophan. Other biogenic amines are made from tyrosine, and include dopamine, norepinephrine, and epinephrine. The chemical epinephrine (epi- = “on”; “-nephrine” = kidney) is also known as adrenaline (renal = “kidney”), and norepinephrine is sometimes referred to as noradrenaline. The adrenal gland produces epinephrine and norepinephrine to be released into the blood stream as hormones.
- Cholinergic system: It is the system based on acetylcholine. This includes the neuromuscular junction as an example of a cholinergic synapse, but cholinergic synapses are found in other parts of the nervous system. They are in the autonomic nervous system, as well as distributed throughout the brain.
- Neuropeptides: These are neurotransmitter molecules made up of chains of amino acids connected by peptide bonds. This is what a protein is, but the term protein implies a certain length to the molecule. Some neuropeptides are quite short, such as met-enkephalin, which is five amino acids long. Others are long, such as beta-endorphin, which is 31 amino acids long. Neuropeptides are often released at synapses in combination with another neurotransmitter, and they often act as hormones in other systems of the body, such as vasoactive intestinal peptide (VIP) or substance P. Other important neuropeptides include those released from the posterior pituitary gland: oxytocin and vasopressin (the latter is also known as anti-diuretic hormone).
The effect of a neurotransmitter on the postsynaptic element is entirely dependent on the receptor protein. First, if there is no receptor protein in the membrane of the postsynaptic element, then the neurotransmitter has no effect. The depolarizing or hyperpolarizing effect is also dependent on the receptor. For example, when acetylcholine binds to a type of receptor called nicotinic receptor, the postsynaptic cell is depolarized. This is because the receptor is a cation channel and positively charged Na+ will rush into the cell. However, when acetylcholine binds to another type of receptor called muscarinic receptor, of which there are several variants, it might cause depolarization or hyperpolarization of the target cell.
On the other hand, the amino acid neurotransmitters, glutamate, glycine, and GABA, are almost exclusively associated with just one effect. Glutamate is considered an excitatory amino acid, but only because Glu receptors in the adult cause depolarization of the postsynaptic cell. Glycine and GABA are considered inhibitory amino acids, again because their receptors cause hyperpolarization.
The biogenic amines have mixed effects. For example, the dopamine receptors that are classified as D1 receptors are excitatory whereas D2-type receptors are inhibitory. Biogenic amine receptors and neuropeptide receptors can have even more complex effects because some may not directly affect the membrane potential, but rather have an effect on gene transcription or other metabolic processes in the neuron.
The important thing to remember about neurotransmitters, and signaling chemicals in general, is that the effect is entirely dependent on the receptor. Neurotransmitters bind to one of two classes of receptors at the cell surface, ionotropic or metabotropic (Figure 17). Ionotropic receptors are ligand-gated ion channels, such as the nicotinic receptor for acetylcholine (allows influx of Na+ cations) or the glycine receptor (allows influx of Cl– anions). A metabotropic receptor involves a complex of proteins that result in metabolic changes within the cell. The receptor complex includes the transmembrane receptor protein, a G protein, and an effector protein. The neurotransmitter, referred to as the first messenger, binds to the receptor protein on the extracellular surface of the cell, and the intracellular side of the protein initiates activity of the G protein. The G protein is a guanosine triphosphate (GTP) hydrolase that physically moves from the receptor protein to the effector protein to activate the latter. An effector protein is an enzyme that catalyzes the generation of a new molecule, which acts as the intracellular mediator of the signal that binds to the receptor. This intracellular mediator is called the second messenger.
Different receptors use different second messengers. Two common examples of second messengers are cyclic adenosine monophosphate (cAMP) and inositol triphosphate (IP3). The enzyme adenylyl cyclase (also called adenylate cyclase; an example of an effector protein) makes cAMP, and phospholipase C is the enzyme that makes IP3. Second messengers, after they are produced by the effector protein, cause metabolic changes within the cell. These changes are most likely the activation of other enzymes in the cell. In neurons, they often modify ion channels, either opening or closing them. These enzymes can also cause changes in the cell, such as the activation of genes in the nucleus, and therefore the increased synthesis of proteins. In neurons, these kinds of changes are often the basis of stronger connections between cells at the synapse and may be the basis of learning and memory.
Sensory receptor specialized for temperature stimuli.
Change in the membrane potential that varies in size, depending on the size of the stimulus that elicits it.
Single process of the neuron that carries an electrical signal (action potential) away from the cell body toward a target cell.
Change in voltage of a cell membrane in response to a stimulus that results in transmission of an electrical signal; unique to neurons and muscle fibres.
End of the axon, where there are usually several branches extending toward the target cell.
Swelling at the end of an axon where neurotransmitter molecules are released onto a target cell across a synapse.
Chemical signal that is released from the synaptic end bulb of a neuron to cause a change in the target cell.
Narrow junction across which a chemical signal passes from neuron to the next, initiating a new electrical signal in the target cell.
Protein molecule that contains a binding site for another specific molecule (called a ligand).
Major region of the diencephalon that is responsible for relaying information between the cerebrum and the hindbrain, spinal cord, and periphery.
Synapse between the axon terminal of a motor neuron and the section of the membrane of a muscle fiber with receptors for the acetylcholine released by the terminal.
Atom with an overall positive or negative charge. Many function as electrolytes.
An amphipathic lipid molecule containing a phosphate head (polar) and two fatty acid tails (non-polar). The major molecule comprising plasma membranes.
"Water loving"; a molecule or portion thereof that is polar and therefore water soluble.
Membrane-spanning protein that has an inner pore which allows the passage of one or more substances (a form of facilitated diffusion).
Form of transport across the cell membrane that requires input of cellular energy.
Difference in the concentration of a substance between two regions.
Nucleotide containing ribose and an adenine base that is essential in energy transfer.
A channel protein (facilitated diffusion) that is activated (opens) when a molecule (such as a neurotransmitter) binds to it.
Ion channel protein (facilitated diffusion) that opens when a physical event directly affects the structure of the protein.
Sense of touch.
Ion channel that opens because of a change in the charge distributed across the membrane where it is located.
Ion channel (facilitated diffusion) that opens randomly and is not gated to a specific event, also known as a non-gated channel.
Distribution of charge across the cell membrane, based on the charges of ions.
Fluid inside cells.
Clear, semi-fluid medium of the cytoplasm, made up mostly of water.
Atom with a negative charge.
Chemical functional group, PO4-, a component of phospholipids and nucleic acids (including ATP).
The difference in voltage measured across a cell membrane under steady-state conditions, typically -70 mV.
Change in a cell membrane potential from rest toward zero.
Return of the membrane potential to its normally negative voltage at the end of the action potential.
Change in cell membrane potential below resting potential (<-70mV).
Time after the initiation of an action potential when another action potential cannot be generated.
Lipid-rich insulating substance surrounding the axons of many neurons, allowing for faster transmission of electrical signals.
Single stretch of the axon insulated by myelin and bounded by nodes of Ranvier at either end (except for the first, which is after the initial segment, and the last, which is followed by the axon terminal).
Gap between two myelinated regions of an axon, allowing for strengthening of the electrical signal as it propagates down the axon.
Slow propagation of an action potential along an unmyelinated axon owing to voltage-gated Na+ channels located along the entire length of the cell membrane.
Quick propagation of the action potential along a myelinated axon owing to voltage-gated Na+ channels being present only at the nodes of Ranvier.
Membrane-bound structure that contains materials within or outside of the cell.
Export of a substance out of a cell by formation of a membrane-bound vesicle.
Small gap between cells in a chemical synapse where neurotransmitter diffuses from the presynaptic element to the postsynaptic element.
Movement of a substance from an area of higher concentration to one of lower concentration.
Molecule (usually a protein) that catalyzes chemical reactions.
Building block of proteins; characterized by an amino and carboxyl functional groups and a variable side-chain.
Signaling molecule released as a neurotransmitter by most postganglionic sympathetic fibres as part of the sympathetic response, or as a hormone into the bloodstream from the adrenal medulla.
Signaling molecule released from the adrenal medulla into the bloodstream as part of the sympathetic response.
Endocrine glands located at the top of each kidney that are important for the regulation of the stress response, blood pressure and blood volume, water homeostasis, and electrolyte levels.
An important neurotransmitter.
Synapse at which acetylcholine is released and binds to the nicotinic or muscarinic receptor.
Functional division of the nervous system that is responsible for homeostatic reflexes that coordinate control of cardiac and smooth muscle, as well as glandular tissue.
A type of covalent bond occurring between amino acids.
Ion with a positive charge.


